Fossil fuels like natural gas, oil, and coal play a major role for the production of energy and commodity chemicals and will continue to do so in the near future. Among them, natural gas, mainly consisting of methane, will become the most important hydrocarbon source owing to its larger reserves and environmentally friendly nature compared to oil and coal. Currently, natural gas is mainly used for home and industrial heating as well as for the generation of electrical power. There is a strong economic interest in developing processes for natural gas conversion to higher-valued products.
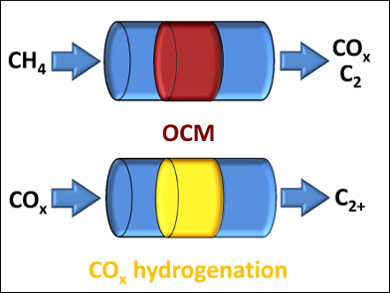
Evgenii V. Kondratenko and colleagues, Leibniz Institute for Catalysis (LIKAT), Rostock, Germany, have developed an approach that combines the oxidative coupling of methane (OCM) reaction with a hydrogenation of COx to higher hydrocarbons. The reactions were carried out in separate reactors and under different reaction conditions. A dual-reactor concept is able to achieve an overall yield of C2+ hydrocarbons of 0.38 at a methane conversion of 0.64.
Compared to two-step processes consisting of syngas generation from methane followed by Fischer-Tropsch synthesis, this concept is more efficient with respect to hydrogen consumption. From an energetic viewpoint, it is also superior, since the OCM reaction is more exothermic than even partial oxidation of methane to syngas.
- Higher Hydrocarbon Production through Oxidative Coupling of Methane Combined with Hydrogenation of Carbon Oxides,
Matthias Albrecht, Uwe Rodemerck, Evgenii V. Kondratenko,
Chem. Ing. Tech. 2014, 86, 894–1900.
DOI: 10.1002/cite.201400066