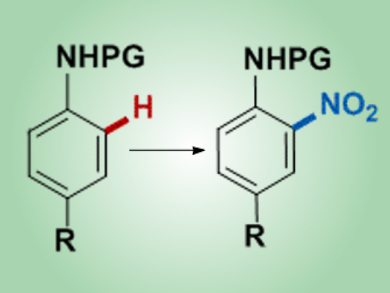
Despite recent advances, a more practical, general, and green method for the catalytic nitration of arenes is desirable. The products of this reaction, nitroarenes, are important intermediates for the synthesis of pharmaceuticals, dyes, and materials and are commonly prepared by the direct electrophilic nitration of arenes under harsh reaction conditions.
Nuria Rodríguez, Ramón Gómez Arrayás, Juan C. Carretero, and co-workers, Universidad Autónoma de Madrid, Spain, have reported a practical copper-catalyzed direct nitration of protected anilines. This procedure uses one equivalent of nitric acid, which produces water as the only stoichiometric byproduct, and is compatible with a variety of N-protecting groups. Remarkable tolerance with respect to the arene substitution was demonstrated and the procedure could also be used to prepare dinitrated aniline derivatives by using two equivalents of nitric acid. Although the mechanism of this reaction is not clear, some experiments are consistent with a radical pathway.
This method can provide rapid access to relevant nitrogen-containing heterocycles for several applications.
- Copper-Catalyzed Mild Nitration of Protected Anilines,
Elier Hernando, Rafael R. Castillo, Nuria Rodríguez, Ramón Gómez Arrayás, Juan C. Carretero,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404000