Nickel-Catalyzed α-Arylation of Ketones with Phenol Derivatives
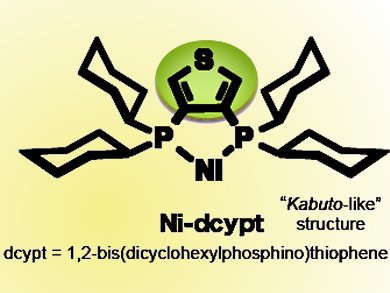
Japanese chemists have developed a new type of nickel catalyst shaped like a Samurai warrior’s “kabuto” helmet. The material accelerates the cross-coupling reaction between carbonyl compounds and readily available phenol derivatives, to form α-arylketones, a framework present in many natural products with physiological and biological activity.
Writing in Angewandte Chemie, Kenichiro Itami, Junichiro Yamaguchi, Ryosuke Takise, and Kei Muto of the Institute of Transformative Bio-Molecules (WPI-ITbM) at Nagoya University, Japan, explain that their catalyst is set to become an important tool in the synthesis of a wide range of versatile alpha-arylketones.
Cross-coupling Reactions Catalyzed by Transition Metals
Transition metal catalysts for use in cross-coupling reactions are well known and have been used in synthetic schemes to access a large number of organic frameworks creating bonds between previously unconnected carbon atoms and so allowing novel structures to be crafted from simpler starting materials. Indeed, such catalysts were celebrated in the 2010 Nobel Prize in Chemistry wherein Richard Heck, Ei-ichi Negishi, and Akira Suzuki shared the award for carbon-carbon bond formation reactions
facilitated by palladium catalysts.
While α-arylation, which joins carbonyl compounds to aromatic rings, is critical in many syntheses, palladium is a rare and costly precious metal. Nickel would be a much cheaper alternative for such reactions and so Itami and colleagues have been hunting for a way to exploit this metal as a catalyst since 2009. As part of this effort the team alighted on phenol derivatives as readily available aryl-coupling partners thus sidestepping aryl halides and the toxic waste products they generate. It is, of course, the tuning of the properties of a transition metal through the addition of ligands that then opens up the conversion and makes it catalytic.
New Catalyst
One such ligand discovered through a screening approach by the team in 2011 is DCYPE (1,2-bisdicyclohexylphosphinoethane). Hooked up to a nickel center, this catalyst can activate phenol derivatives.
Unfortunately, it does not initiate the cross-coupling between carbonyl compounds and phenol derivatives. A chemical tweak in the form of adding a sulfur-containing thiophene ring to make DCYPT (1,2-bisdicyclohexylphosphinothiophene) would unlock nickel’s potential. The ligand is air-stable unlike its predecessor or indeed other related ligands, which is important for experimental simplicity.
The team reports that the catalyst helps initiate a high-yielding reaction, 77 % yield achievable on naphthalen-2-yl dimethylcarbamate for instance. DCYPT also has the advantage of working with weak bases which means the catalyst will work with many more starting materials than it otherwise would. Indeed, the presence of a base is essential to this reaction. They demonstrated proof of principle with numerous phenol pivalates, such as those derived from 6-quinolinol, 6-methoxycarbonyl-2-naphthol and 4-functionalized phenols which could all be coupled with a 2-phenylacetophenone to give the corresponding α-arylated products, allowing unnatural amino acids and peptides to be synthesized, for example.
The team used X-ray crystallography to characterize the intermediate in what they assume is a carbon-oxygen oxidative addition reaction that takes place in order to reconcile the reaction mechanism. The researchers suggest that the reaction proceeds through a catalytic Ni0/NiII redox cycle in which CO oxidative addition of the phenol derivative on to the Ni0/DCYPT complex occurs to form an Ar–Ni–OR complex. C–H nickelation of a ketone with base gives a diorgano-nickel(II) complex and then the coupling product is eliminated reductively with regeneration of the Ni0 catalyst.
Future Outlook
“To tame a hot nickel metal, we perhaps need to re-tune the ligand structure”, Itami told ChemistryViews.org. “It might take some time, but as we experienced so many times, we should be able to have a very active and general catalyst when tuning the reactivity of nickel properly.” He adds that they will also pursue application to a broader class of carbonyl compounds, the development of enantioselective catalytic system, and the activation of unactivated sp3 C–H bonds.
“Toxicity and practicality aside, the means to utilize aryl esters as coupling partners represents a major conceptual advance in the search for alternatives in the cross-coupling arena”, Rubén Martín of the Institute of Chemical Research of Catalonia, Tarragona, Spain, told ChemistryViews.org. “The Ni-catalyzed α-arylation of aryl pivalates developed by Itami and co-workers undoubtedly opens up new perspectives in synthetic organic chemistry.” Martín adds that the work importantly also sheds light on the mechanism by which cleavage of C–O bonds in aryl esters takes place. “Itami’s work will inspire others to develop even more applicable Ni-catalytic cross-coupling reactions, either within the field of α-arylation or other related cross-coupling events”, he adds.
- Nickel-Catalyzed α-Arylation of Ketones with Phenol Derivatives,
R. Takise, K. Muto, J. Yamaguchi, K. Itami,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201403823