Twisted polycyclic aromatic hydrocarbons are a class of attractive molecules in terms of their structural uniqueness and intriguing optoelectronic properties. Synthesis of a broad range of such compounds remains a challenging issue.
Hayato Tsuji, Eiichi Nakamura, and co-workers, University of Tokyo, Japan, report selective synthesis of a variety of functional dibenzo[g,p]chrysenes (DBCs). It is based on regio- and stereoselective stannyllithiation reaction of alkynes, which they have originally developed, followed by cross-coupling reaction and intramolecular dehydrobromination.
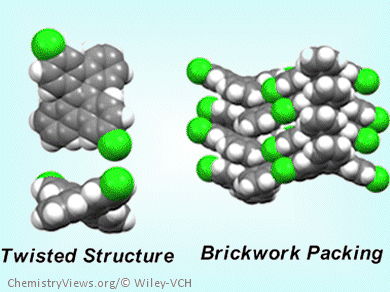
The DBC derivatives possess brickwork packing in single crystals. These originate from their twisted DBC core structure. Some of the functionalized DBCs exhibit ambipolar and high charge carrier mobility up to the order of 10–3 cm2V–1s–1 in amorphous state, which is significantly higher than the parent DBC.
Taking advantage of these compounds’ unique structures and properties, the researchers expect application to optical and electronic devices.
- Synthesis, Crystal Packing, and Ambipolar Carrier Transport Property of Twisted Dibenzo[g,p]chrysenes,
Yasuyuki Ueda,Hayato Tsuji, Hideyuki Tanaka, Eiichi Nakamura,
Chem. Asian J. 2014.
DOI: 10.1002/asia.201402102



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)