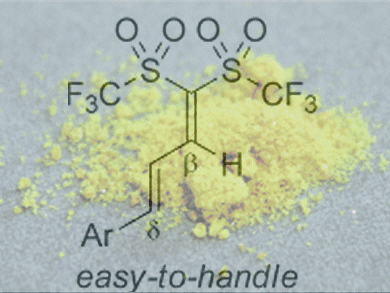
Although the acidities of common carbon acids are too weak to catalyze some organic reactions, 1,1-bis(triflyl)alkanes bearing an acidic C–H moiety exceptionally are potent acid catalysts due to their strong acidity, which is enhanced by gem substitution of the carbon atom by two triflyl groups. Hikaru Yanai and co-workers, Tokyo University of Pharmacy and Life Sciences, Japan, have developed an effective method for synthesizing this type of carbon acid.
They found that 1,1-bis(triflyl)alkadienes, which are easily prepared by mixing bis(triflyl)methane and α,β-enals, can be used as stable but useful building blocks for the carbon acids. For example, the reaction of alkadienes with organocerium reagents prepared from Grignard reagents and anhydrous CeCl3 smoothly yielded β-branched carbon acids after acidic workup. Likewise, NaBH4 reduction gave the nonbranched carbon acids.
Some carbon acids thus obtained are reasonably active catalysts in organic reactions, such as Mukaiyama aldol reactions. Therefore, the present result provides a convenient synthesis of bis(triflyl)alkanes and verifies that organic acids containing a bis(triflyl)methyl group can become a new class of acid catalysts.
- 1,1-Bis(triflyl)alkadienes: Easy-To-Handle Building Blocks for Strongly Acidic Carbon Acids,
Hikaru Yanai, Saki Egawa, Kenta Yamada, Junpei Ono, Motohide Aoki, Takashi Matsumoto, Takeo Taguchi,
Asian J. Org. Chem. 2014, 3, 556–563.
DOI: 10.1002/ajoc.201402010