Turning Waste into Raw Materials
No one who has cracked open a rotten egg will forget its infernal stench. Biofuel plants, sewage treatment plants, and petroleum refineries can generate substantial amounts of foul-smelling hydrogen sulfide gas, which is highly toxic at higher concentrations. In the journal Angewandte Chemie, a team of Australian and Chinese researchers has now introduced an innovative photoelectrochemical process in which solar energy is used to split this undesirable by-product into sulfur and hydrogen, converting it into a source of raw materials.
A variety of techniques have been used to remove hydrogen sulfide (H2S) from polluted exhaust gases and occasionally put it to further use. While sulfur can be extracted in some processes, the hydrogen cannot. This is unfortunate because hydrogen is actually an important energy source for future fuel-cell technology.
Unfortunately, it isn’t possible to split H2S to gain hydrogen and sulfur simultaneously. Approaches using photochemical splitting seem particularly attractive because solar energy could be used to meet the high energy demand of this reaction. However, no ecologically and economically feasible process has been found to date. This could now change thanks to a new approach developed by a team headed by Lianzhou Wang, University of Queensland, Australia, and Can Li, Chinese Academy of Sciences and Dalian Laboratory for Clean Energy, China.
Photochemical–Chemical Loop
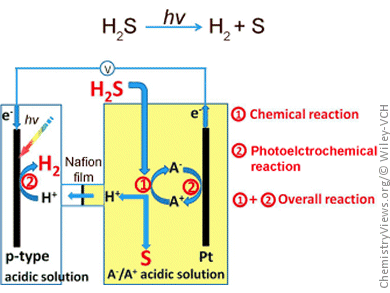
Their success lies in a photochemical–chemical loop whose reactions are coupled through a redox pair. A redox pair is a combination of the reduced and oxidized form of the same element that can easily be interconverted. For their process, the researchers used either divalent and trivalent iron ions (Fe2+/Fe3+) or the iodide/triiodide (I−/I3−) system.
The hydrogen sulfide gas is introduced into the electrolyte of the anodic compartment of an electrochemical cell. Here, a chemical reaction causes it to be bound to the oxidized form of the redox pair (which is thus reduced) and converted into sulfur, which precipitates out as a yellow solid, and hydrogen cations (protons). The protons can pass through the semipermeable membrane that separates the anodic and cathodic compartments. The second reaction is photoelectrochemical: as protons are reduced at the cathode by taking up electrons, the reduced form of the redox pair is returned to its oxidized state by giving up electrons at the anode. The driving force for this is sunlight, which generates “electron–hole pairs” at the photoanode. These holes can then be filled by the absorbed electrons.
The redox pairs continuously cycle between the oxidized and reduced forms so that the overall reaction is the splitting of hydrogen sulfide into sulfur and hydrogen by sunlight.
- An Integrated Photoelectrochemical–Chemical Loop for Solar-Driven Overall Splitting of Hydrogen Sulfide,
Xu Zong, Jingfeng Han, Brian Seger, Hongjun Chen, Gaoqing (Max) Lu, Can Li, Lianzhou Wang,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201400571



Will the precipitated sulfur solid buildup on the anode and form a coating on the anode? Would such a sulfur coating barrier stop or slow the reactions?