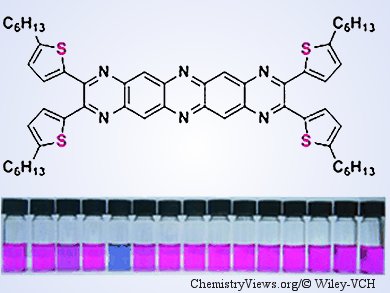
As one of most abundant essential trace elements in the human body, copper plays a critical role as a catalytic cofactor for a variety of metalloenzymes. However, a relatively high level of copper in the body can cause liver or kidney damage. Although a number of fluorescent Cu2+ sensors have been reported, the selectivity for Cu2+ over other metals ions, such as Fe3+ or Pb2+, is not very satisfactory. In addition, most traditional probes to sense Cu2+ through a “turn-off” mechanism due to the paramagnetic nature of Cu2+ ions are not practical. Therefore, developing a method for highly selective and sensitive detection of copper is necessary through a “turn-on” response is desirable.
Qichun Zhang and co-workers from Nanyang Technological University, Singapore, have developed the “turn-on” Cu2+ chemosensor 1,2,5,6-tetra(5-hexylthiophene-2-yl)-hexaazapentacene (4HP) through a one-step cyclocondensation. The binding behavior of 4HP toward various cations demonstrated a selective and sensitive response towards Cu2+ ions. The detection limit is 1.2 µM in DMF solution. The group hopes that the work will provide a blueprint for future development of metal-ion sensors.
- Synthesis, Characterization, and Sensing Behavior of an N-heteropentacene,
Gang Li, Junkuo Gao, Qichun Zhang,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300210