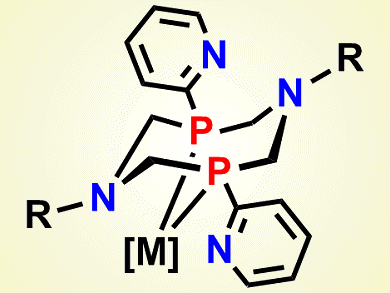
New pyridylphosphines based on the cyclic aminomethylphosphine platform, combining the properties of the two systems, pyridylphosphine and aminomethylphosphine, have been developed by Elvira I. Musina, Russian Academy of Sciences, Kazan Scientific Center, Russia, and colleagues. The introduction of ortho-pyridyl substituents expands the coordination modes of these ligands by introduction of an additional intramolecular pendant base. It is situated in close proximity to the first coordination sphere of the corresponding transition metal.
The researchers report the synthesis and crystal structures of several 1,5-diaza-3,7-diphosphacyclooctanes with ortho-pyridyl substituents on the phosphorus atoms, their neutral P,P-chelate complexes with platinum(II), palladium(II), and tungsten(0), and their cationic bis-P,P-chelate complexes with platinum(II), palladium(II), and nickel(II). They also found an improvement in the catalytic activity of the cationic nickel(II) bis-P,P-chelate complexes in both electrochemical oxidation and hydrogen evolution. In addition, they found that inclusion of the nickel complexes into the near-electrode layer increased the power density of a micro fuel cell.
- New Functional Cyclic Aminomethylphosphine Ligands for the Construction of Catalysts for Electrochemical Hydrogen Transformations,
E. I. Musina, V. V. Khrizanforova, I. D. Strelnik, M. I. Valitov, Y. S. Spiridonova, D. B. Krivolapov, I. A. Litvinov, M. K. Kadirov, P. Lönnecke, E. Hey-Hawkins, Y. H. Budnikova, A. A. Karasik, O. G. Sinyashin,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201304234