Münchnone is a known five-membered cyclic dipole that is traditionally used in cycloaddition reactions with alkyne and alkene dipolarophiles.
A joint research team led by Richard C. Larock, Iowa State University, Ames, IA, USA, and Feng Shi, Henan University, Kaifeng, China, uncovered that münchnones exhibit excellent reactivity when arynes are used as the dipolarophile.
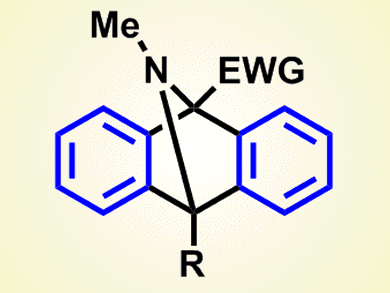
The reaction proceeds through a series of cycloaddition events and eventually affords 9,10-dihydro-9,10-epiminoanthracenes. Although the products are traditionally prepared from isoindoles by using the same chemistry as the last step in the new process, the preparation of isoindole derivatives can be quite difficult.

Münchnones can be easily obtained from amino acids, and thus the aryne–münchnone chemistry appears an ideal route to this type of product.
 Aryne Cycloaddition with Stable Münchnones: Synthesis of 9,10-Dihydro-9,10-epiminoanthracenes and Isoindoles,
Aryne Cycloaddition with Stable Münchnones: Synthesis of 9,10-Dihydro-9,10-epiminoanthracenes and Isoindoles,
Yuesi Fang, Richard C. Larock, Feng Shi,
Asian J. Org. Chem. 2014, 3, 55–57.
DOI: 10.1002/ajoc.201300221