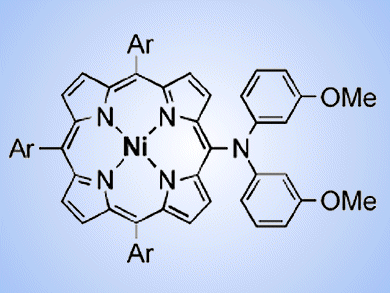
PEPPSI (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) is not a drink, but the best catalyst to quench materials chemists’ thirsts for new organic functional materials. Since triarylamines, including meso-aminoporphyrins, have found wide applications in organic electronics, such as organic light-emitting devices and organic solar cells, organic chemists have been developing efficient amination reactions. However, aminations of bulky aryl halides are still difficult.
This issue drove Atsuhiro Osuka, Hideki Yorimitsu, and co-workers, Kyoto University, Japan, to develop a new amination technology. The group reported palladium-catalyzed aminations of meso-bromoporphyrins (example pictured) and 9-haloanthracenes with diarylamines. The Pd–PEPPSI complex, which was originally developed by Michael Organ, York University, Toronto, ON, Canada, and is now commercially available and easy to handle, has the best catalytic activity. A wide variety of diarylamines, such as diphenylamine, phenoxazine, phenothiazine, 9,10-dihydroacrydine, and carbazole, are applicable in the reactions.
- Amination of meso-Bromoporphyrins and 9-Haloanthracenes with Diarylamines Catalyzed by a Palladium-PEPPSI Complex,
Yuko Suzuki, Norihito Fukui, Kei Murakami, Hideki Yorimitsu, Atsuhiro Osuka,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300162