Various CO2 capture and storage (CCS) technologies, such as amine scrubbers and cryogenic separation, are already available, but the associated large energy penalties limit large-scale applications. Many of the alternative solid adsorbent CO2-capture materials adsorb CO2 by chemisorption, thus making regeneration of the adsorbent material energy intensive.
Some polar functional groups have been shown to enhance affinity of porous materials for CO2 because of the high quadrupole moment and polarizability of CO2. Adsorbents with these functional groups are advantageous because chemisorption is not involved and so they can be readily regenerated after CO2 adsorption.
Although metal—organic framework (MOF) materials are well-studied solid adsorbents, they are often unstable towards moisture. Porous organic polymers (POPs), on the other hand, generally show good mechanical, thermal, and chemical stability owing to the covalent nature of the bonding networks.
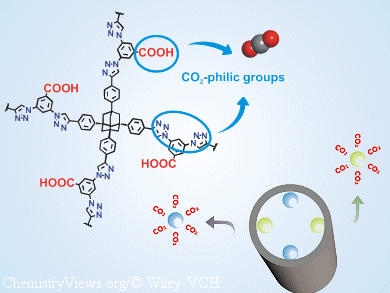
Myunghyun Paik Suh and Lin-Hua Xie, Seoul National University, Korea, have synthesized a new POP (SNU-C1) that contains two types of CO2-attracting groups, that is, carboxy and triazole groups. This POP has a high CO2-uptake capacity at room temperature, high selectivity, high regenerability, and good stability towards water. After activation by the conventional evacuation or supercritical-CO2 drying methods, the CO2-capture ability of both guest-free materials was evaluated by experimental sorption studies and calculation of five vacuum-swing adsorption separation parameters.
These studies revealed that the porosity and CO2-capture performance of these POPs depend upon the method of activation.
- High CO2-Capture Ability of a Porous Organic Polymer Bifunctionalized with Carboxy and Triazole Groups,
Lin-Hua Xie, Myunghyun Paik Suh,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201301822