Expanded porphyrins have attracted considerable attention because of their versatile electronic and structural properties. Furthermore, they show great promise for applications such as near-IR absorbing dyes, or as sensors for anions or explosives.
Hexaphyrin acts as a benchmark for expanded porphyrins and has given rise to a compilation of intriguing chemistry, such as rich coordination chemistry and skeletal rearrangements. However, further examples of modifications are still lacking.
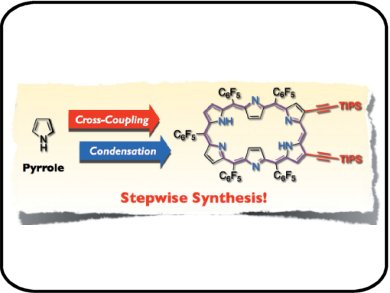
Atsuhiro Osuka and colleagues, Kyoto University, Japan, report a bottom-up, stepwise synthetic pathway that provides peripherally functionalized hexaphyrins by using prefunctionalized dipyrromethane Sn4+ complexes. Furthermore, this approach also allows the preparation of expanded hexaphyrins that have unstubstituted meso carbon atoms. This approach is appealing as hexaphyrins with unsubstituted meso carbon atoms make other reactions possible, such as oxidations to gain stable radicals.
Therefore, this bottom-up synthesis highlights the candidacy of hexaphyrins for further research.
- Bottom-Up Synthesis of Bis(triisopropylsilyl)ethynyl Hexaphyrin Bearing an Unsubstituted meso-Carbon,
H. Mori, K. Naoda, A. Osuka,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300061