Rhodesain – A Target for Drug Candidates
Sleeping sickness kills tens of thousands of people in Africa each year. Current chemotherapies are subject to various limitations, including resistance. Rhodesain, an enzyme of the parasites that cause this illness (human African trypanosomiasis), has emerged as a target for new drug candidates. Scientists led by F. Diederich, ETH Zürich, Switzerland, studied the molecular recognition properties of rhodesain and developed a series of triazine nitrile inhibitors as lead compounds using structure-based molecular modeling.
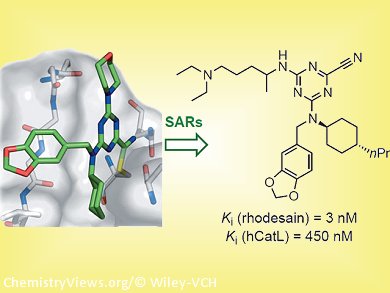
As they report in ChemMedChem, the team composed of researchers from ETH Zürich and the groups of Tanja Schirmeister, University of Mainz, Germany, David W. Banner, F. Hoffmann-La Roche, Switzerland, and the Swiss Tropical and Public Health Institute systematically analyzed the binding preferences for each of the pockets in the active site of rhodesain, obtaining inhibitors with affinities (Ki values) down to the single-digit nanomolar range. They were able to effect inhibition of the proliferation of cultured parasites at the sub-micromolar level with inhibitors bearing a basic substituent, leading to a 35-fold increase in antitrypanosomal activity.
Imidazopyridine-based Inhibitor
“To control the activation of the inhibitor’s nitrile head group, we envisioned a central scaffold similar to natural ones and based on imidazopyridine, which allowed a pronounced reduction of the nitrile electrophilicity, while simultaneously maintaining nanomolar affinity for rhodesain,” says Diederich. The triazine nitrile head group had been problematic in causing off-target effects with other nucleophiles present in the cell. X-ray crystal structures of human cathepsin L, a close structural relative of rhodesain, both in complex with a triazine nitrile inhibitor and in its apo form, confirmed the proposed binding mode of the ligand series and provided important insight into the hydration state of the active site.
These results of this research provide the basis for further optimization studies on rhodesain, focusing on imidazopyridine nitrile-derived ligands with improved enzymatic and cell-based activity.
- Optimization of Triazine Nitriles as Rhodesain Inhibitors: Structure–Activity Relationships, Bioisosteric Imidazopyridine Nitriles, and X-ray Crystal Structure Analysis with Human Cathepsin L,
Veronika Ehmke, Edwin Winkler, David W. Banner, Wolfgang Haap, W. Bernd Schweizer, Matthias Rottmann, Marcel Kaiser, Céline Freymond, Tanja Schirmeister, François Diederich,
ChemMedChem 2013.
DOI: 10.1002/cmdc.201300112