The activities and selectivities of transition metal complex catalysts are affected by both the steric and the electronic effects of the ligands. Biaryl phosphite ligands are extensively used in homogeneous catalysis. Therefore, it is important to understand the factors that affect their steric and electronic properties.
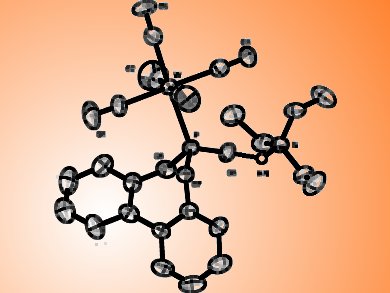
Gary M. Gray, University of Alabama at Birmingham, AL, USA, and colleagues have prepared tungsten(0) pentacarbonyl complexes with chlorophosphite ligands derived from either 2,2′-biphenol or (±)-1,1′-bi-2-naphthol. Thay have hydrolyzed these coordinated ligands to generate tungsten(0) pentacarbonyl complexes with the corresponding phosphonous acid ligands. The electron donor/acceptor properties of the ligands in these complexes have been evaluated by multinuclear NMR spectroscopy, and the steric properties of the ligands by X-ray crystallography.
The identity of the biaryl group does not affect the steric or the electronic properties of the ligands, as changing the biaryl groups from biphenyl to binaphthyl showed no affect the electron-donor ability of the ligand, whereas changing the third substituent from chloro to oxo has a significant effect. Also neither changing the biaryl group from biphenyl to binaphthyl nor replacing the chloro substituent with an oxo substituent that is hydrogen bonded to a triethylammonium group have a significant effect on the cone angles. In addition, important intra- and intermolecular interactions that favor certain ligand conformations have been identified.
The data could be useful for the development of catalytic structure–activity relationships that could be used in the rational design of catalysts.
- Multinuclear NMR Spectroscopic and X-ray Crystallographic Studies of Electronic and Steric Effects of Phosphonous Acid Ligands and Their Chlorophosphite Ligand Precursors in Tungsten Pentacarbonyl Complexes,
Samantha D. Hastings, Houston Byrd, Leanne N. Gray, Michael J. Jablonsky, Jason L. Freeman, Gary M. Gray,
Eur. J. Org. Chem. 2013.
DOI: 10.1002/ejic.201300121