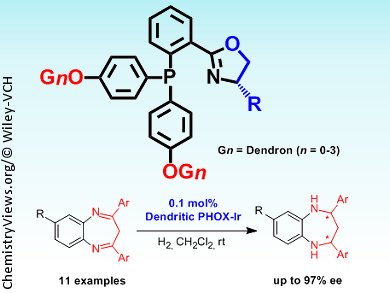
Iridium catalysts with chiral P,N-ligands catalyze asymmetric hydrogenation reactions, but catalyst dimerization and consequent deactivation is problematic. Researchers in China have used a dendritic catalyst to suppress dimerization and increase activity in the hydrogenation of benzodiazepines.
Qing-Hua Fan and co-workers, Institute of Chemistry, Chinese Academy of Sciences, Beijing, attached dendritic poly(benzyl ether) arms to a chiral phosphinooxazoline core (pictured). Complexation of the dendrimers with iridium led to an active catalyst for the asymmetric hydrogenation of 2,4-diaryl-1,5-benzodiazepines. The dendritic catalyst gave high conversion with good diastereo- and enantioselectivity and showed better catalytic performance than its small-molecule counterpart, which suffered from the formation of inactive dimers and trimers. The catalyst could be recovered easily by precipitation with hexane, although subsequent runs with the same catalyst showed lower conversion.
The positive dendritic effect holds promise for the development of related catalysts for other transformations.
- Highly Enantioselective Hydrogenation of 2,4-Diaryl-1,5-Benzodiazepines Catalyzed by Dendritic Phosphinooxazoline Iridium Complexes,
Baode Ma, Ziyuan Ding, Ji Liu, Yanmei He, Qing-Hua Fan,
Chem. Asian J. 2013.
DOI: 10.1002/asia.201300150