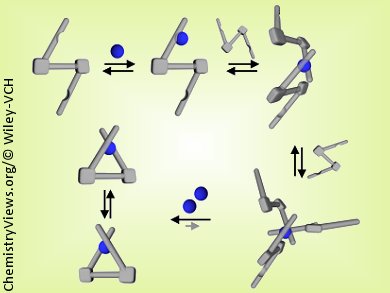
Dongwhan Lee and his group at Indiana University, Bloomington, USA, have shown that what appear to be straightforward spectroscopic features obtained by UV/Vis and fluorescence spectroscopy, tools often used to study metal–ligand assembly, could provide an oversimplified and inaccurate picture of the actual equilibrium between metal and ligand in solution. They have discovered that exciton-coupled circular dichroism (ECCD) spectroscopy provides a useful spectroscopic handle to understand the otherwise “invisible” solution dynamics.
By using ECCD as a structure-sensitive spectroscopic tool, they have experimentally confirmed for C2-symmetric chiral chelates: 1) the reaction pathways of metal–ligand assembly involving multiple, slowly exchanging species; 2) the thermodynamic stability provided by the conformationally rigid and π-conjugated ligand skeleton; and 3) the kinetic lability responsible for the P-to-M (and vice versa) helicity switching and the mechanistic implications of the energy barrier.
- Stereodynamics of Metal–Ligand Assembly: What Lies Beneath “Simple” Spectral Signatures of C2-Symmetric Chiral Chelates,
J. Jung, J. Jo, M. Laskar, D. Lee,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201204216