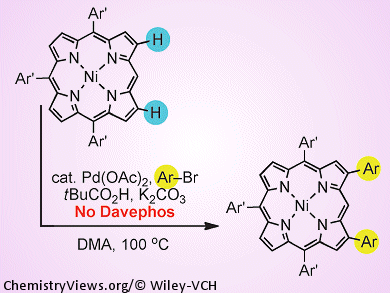
It all started with the revelation that the phosphine ligand 2-dicyclohexylphosphino-2′-(N,N-dimethylamino) biphenyl, which is better known by its alias DavePhos, is not necessary for directly coupling an aryl group with benzene. That led Atsuhiro Osuka, Hideki Yorimitsu, and co-workers, Kyoto University, Japan, to ask the same question of some of their reactions: is DavePhos really necessary in arylation reactions with porphyrin cores?
As it turns out, the answer is no. Palladium-catalyzed arylation of a nickel-coordinated porphyrin proceeds just as well, if not better, without DavePhos in the reaction mixture as it does when the extra ligand is present. Reducing reagent use is good news in terms of costs, environmental impact, and operational simplicity, and the team now intends to use the new conditions to create new porphyrin derivatives at a lower cost to everyone.
- Direct Arylation of Porphyrins with π-Extended Aryl Bromides under Ligand-free Fagnou–Hartwig Conditions,
Yutaro Yamamoto, Sumito Tokuji, Takayuki Tanaka, Hideki Yorimitsu, and Atsuhiro Osuka
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201200198