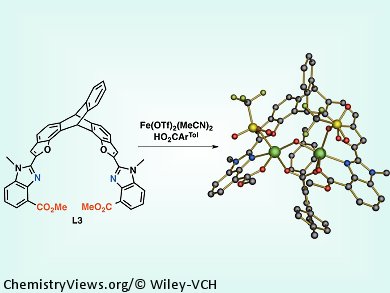
Small-molecule models of the active sites of diiron enzymes help in elucidating the mechanism of enzyme action in biological systems and they can also be used as effective catalysts. The closer their structure resembles the natural compound, the more similar are their function as catalysts to that of natural enzymes. Enzyme action depends highly on the structure and relative orientation of constituents of the enzyme molecule. In natural systems, the proper orientation of coordinating residues is dictated by the predefined conformation of the folded protein. In small-molecule models, however, the appropriate ligand must be selected.
Stephen Lippard and co-workers, Massachusetts Institute of Technology (MIT), USA, report an advanced design of highly preorganized ligands for preparing biomimetic models of the diiron active site of soluble methane monooxygenase hydroxylase (sMMOH). The team’s new ligand has a triptycene backbone containing benzoxazole arms with more nucleophilic benzimidazole donors instead of the previously used benzoxazole substituents. The new ligand is more effective in binding to iron and can more accurately replicate the structural and electronic properties of O2-activating carboxylate-bridged diiron centers found in biological systems.
- Triptycene-Based, Carboxylate-Bridged Biomimetic Diiron(II) Complexes,
Yang Li, Chan Myae Myae Soe, Justin J. Wilson, Suan Lian Tuang, Ulf-Peter Apfel, Stephen J. Lippard,
Eur. J. Inorg. Chem. 2013.
DOI: 10.1002/ejic.201201387