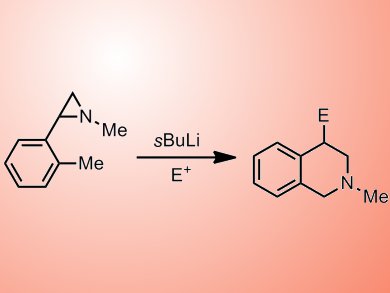
Renzo Luisi and co-workers have reported an interesting approach for the formation of tetrahydroisoquinoline (THIQ) derivatives. Their procedure involves treatment of an aziridine precursor (such as 1) with sBuLi to generate intermediate 2 which can then be used in the formation of functionlized aziridines or C4-substituted THIQs (green and blue arrows respectively, see scheme 1). During optimization of the reaction conditions, the team found that temperature played an important role in determining which products were formed; at lower temperatures (–48 °C) functionlized aziridines were formed preferentially whereas allowing the reaction to warm to 0 °C after lithiation led to THIQ products in modest to good yields. The group opted to use a flow microreactor to achieve the best thermal control.

The procedure was also extended to aziridine precursors bearing an allyl group (3) to form vinyl substituted THIQs, although a slightly higher temperature of 60 °C was required (scheme 2).

Based on these results it may be interesting to replace the allyl group with another which can stabilize a negative charge (a simple phenyl group for example) to further extend the substrate scope.
- Synthesis of 1,2,3,4-Tetrahydroisoquinolines by Microreactor-Mediated Thermal Isomerization of Laterally Lithiated Arylaziridines,
Arianna Giovine, Biagia Musio, Leonardo Degennaro, Aurelia Falcicchio, Aiichiro Nagaki, Jun-ichi Yoshida, Renzo Luisi,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201203533