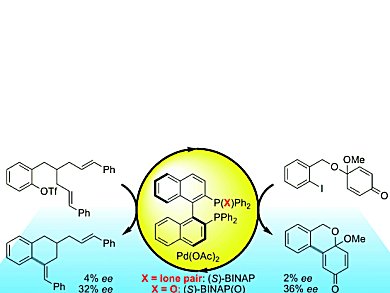
The Mizoroki-Heck reaction is widely used for the enantioselective synthesis of sterically strained molecules with tertiary or quaternary carbon centers. The chiral phosphine ligand 2,2,’-bis(diphenylphosphino)-1,1,’-binaphthyl (BINAP) has been the running favorite for this reaction. However, in desymmetrizing Mizoroki-Heck cyclizations, the use of BINAP yields nearly racemic material. For these reactions, Martin Oestreich and Thorsten H. Wöste, Technische Universität Berlin, Germany, have uncovered the enantioselectivity of BINAP(O) as a palladium ligand, which contains an oxidized phosphine center P=O.
BINAP(O) induced unprecedented enantiocontrol. Up to 18-fold increases in enantioselectivity with yields of up to 94 % were achieved for the cyclizations with BINAP(O) as a chiral ligand compared to those with the conventional BINAP ligand.
BINAP(O) acts as a hemilabile chiral ligand, allowing for reversible coordination of the weak donor P=O group. As a bidentate ligand, BINAP(O) mediates the equilibration of diastereomeric alkene-palladium(II) complexes. Its subsequent dissociation from the palladium(II) atom renders it monodentate, which is known to be advantageous to the ring-closing reaction. These results have great implications for the scope of enantioselective cyclization reactions, putting BINAP(O) on the map!
- Hemilabile BINAP(O) as a Chiral Ligand in Desymmetrizing Mizoroki–Heck Cyclizations,
T. H. Wöste, M. Oestreich,
ChemCatChem 2012, 4, 2096–2101.
DOI: 10.1002/cctc.201200300