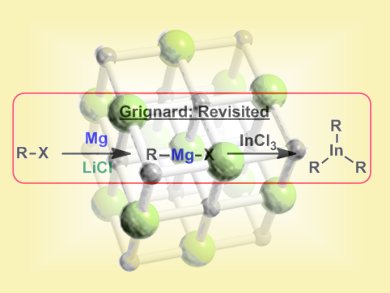
The importance of organomagnesium species in synthetic chemistry is difficult to overstate as they are involved in several C–C bond forming reactions. Important examples include addition of Grignard reagents to carbonyl groups or their use in cross-coupling reactions, e.g., Kumada coupling. Over the last decade, Paul Knochel and co-workers, Ludwig-Maximilians University, Munich, Germany, have reinvestigated the insertion of magnesium into organic halides originally pioneered by Victor Grignard over 100 years ago. Their studies revealed LiCl is a powerful additive for promoting the formation of organomagnesium species under very mild conditions.
The team now report that combining an organohalide, magnesium, and LiCl in the presence of InCl3 leads to the formation of triorganoindium species which can be used in subsequent palladium catalyzed cross-coupling reactions. Organoindium formation proceeds under mild conditions (THF, 25 °C, ~4 h) and tolerates a wide variety of functional groups. Similarly, the cross coupling reactions can be performed with a broad range of aryl halides. Some representative examples of the overall process are shown below:

The role of the LiCl, it is suggested, is to prevent the formation of polymeric aggregates of the Grignard reagent, leading to higher reactivity. The combination of broad substrate scope and mild conditions mean this procedure will surely find further applications in organic synthesis.
- Preparation of Functionalized Organoindium Reagents by means of Magnesium Insertion into Organic Halides in the Presence of InCl3 at room temperature,
S. Bernhardt, Z.-L. Shen, P. Knochel,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201203795
Also of interest:
- A LiCl-Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl- and Heteroarylmagnesium Compounds from Organic Bromides,
Arkady Krasovskiy, Paul Knochel,
Angew. Chem. Int. Ed. 2004, 43, 3333–3336.
DOI: 10.1002/anie.200454084 - Convenient Preparation of Polyfunctional Aryl Magnesium Reagents by a Direct Magnesium Insertion in the Presence of LiCl,
Fabian M. Piller, Prasad Appukkuttan, Andrei Gavryushin, Matthew Helm, Paul Knochel,
Angew. Chem. Int. Ed. 2008, 47, 6802–6806.
DOI: 10.1002/anie.200801968 - EROS Best Reagent Award 2011,
ChemViews magazine 2012.
Paul Knochel has won the EROS Best Reagent Award 2011 for the development of Turbo-Grignard™