Molecular switches can be switched between conformational states in the gas phase or in solution. However, such molecules cannot be switched when adsorbed on surfaces unless they are functionalized in a certain way so that the photochromic moiety is decoupled from the substrate.
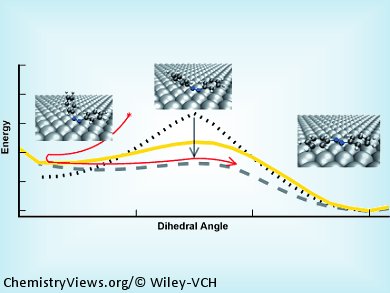
Reinhard J. Maurer and Karsten Reuter, Technical University München, Germany, have examined this phenomenon. They hypothesized that the lack of switching on surfaces arose from a change in ground-state stability, and used dispersion-correlated DFT (density functional theory) calculations to investigate the behavior of surface adsorption on the ground-state barriers of azobenzene.
The calculations showed that surface adsorption of azobenzene results in lowering of the ground state barriers and thus loss of bistability, as immediate thermal reisomerization to the original state occurs. In order to achieve switching of such molecules on surfaces, the authors conclude that molecular functionalization should result in an interaction that minimizes excited-state quenching and that all the molecular geometries related to the switching process need to have a balanced interaction with the surface, for example, by selective destabilization of the diradical rotational transition state, or by reducing the electron availability of the substrate.
- Bistability Loss as a Key Feature in Azobenzene (Non-)Switching on Metal Surfaces,
R. J. Maurer and K. Reuter,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201205718 - Angew. Chem. 2012.
DOI: 10.1002/ange.201205718