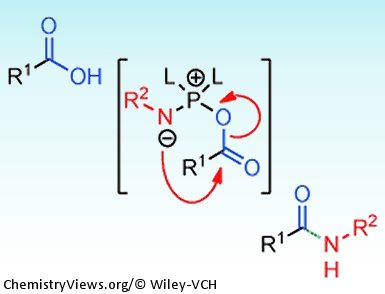
Brandon Ashfeld and co-workers, University of Notre Dame, USA, have recently reported a new method for the formation of amides. Their strategy involves the use of a chlorophosphite reagent to simultaneously activate a carboxylic acid and an azide which promotes a “traceless Staudinger ligation” (see scheme).

This transformation boasts two key-features:
- As the key acyl substitution step is intramolecular, occurring after formation of intermediate A, the reaction can be performed in the presence of other electrophilic or nucleophilic functional groups.
- Reaction byproducts and unreacted starting material can be conveniently separated from the product by aqueous work-up. Anyone who has ever struggled to remove trace amounts of ureas from carbodiimide promoted coupling reactions will certainly appreciate this added bonus.
In a follow up publication, the team demonstrates how the transformation can also be achieved using catalytic triphenylphosphine and a stoichiometric amount of a silane reductant. The team also demonstrated examples of the reaction’s application in lactam and peptide bond formation, making this a highly useful synthetic reaction.
- Direct Acyl Substitution of Carboxylic Acids: A Chemoselective O- to N-Acyl Migration in the Traceless Staudinger Ligation,
A. D. Kosal, E. E. Wilson, B. L. Ashfeld,
Chem. Eur. J. 2012, 18(45), 14444–14453.
DOI: 10.1002/chem.201201773 - Phosphine Based Redox Catalysis in the Direct Traceless Staudinger Ligation of Carboxylic Acids and Azides,
A. D. Kosal, E. E. Wilson, B. L. Ashfeld,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201206533
Also of interest:
- Synthesis of Amides from Esters and Amines with Liberation of H2 under Neutral Conditions,
Boopathy Gnanaprakasam and David Milstein,
J. Am. Chem. Soc. 2011, 133, 1682–1685.
DOI: 10.1021/ja109944n - Direct Synthesis of Amides from Alcohols and Amines with Liberation of H2,
Chidambaram Gunanathan, Yehoshoa Ben-David, David Milstein,
Science 2007, 317, 790–792.
DOI: 10.1126/science.1145295