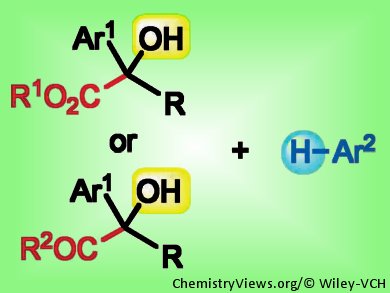
α-Quaternary carbonyl compounds have numerous applications in organic synthesis, medicinal research, and catalyst
design, but are usually difficult to synthesize. Jian Zhou and Long Chen, East China Normal University, Shanghai, have developed a catalytic Friedel–Crafts arylation of tertiary α-hydroxyesters and α-hydroxyketones to fill this gap. Their method uses metal perchlorate hydrates or perchloric acid to catalyze the addition of various arenes and heteroarenes, such as 2-methylthiophene, 2-methylfuran, anisole, and a variety of substituted indoles, yielding α-quaternary carbonyl compounds.
The aqueous HClO4 catalyst is cheap and easy to handle, and the reaction is tolerant to a variety of functional groups. These advantages, together with the relatively mild conditions employed – a few minutes to hours at 60 °C and 1–5 mol % catalyst – suggest that the method is promising for broad application in organic synthesis. Moreover, it is straightforward to convert the resulting carbonyl compounds into, for example, the corresponding acid by hydrolysis or the alcohol by reduction.
- A Highly Efficient Friedel–Crafts Reaction of Tertiary α-Hydroxyesters or α-Hydroxyketones to α-Quaternary Esters or Ketones,
Long Chen, Jian Zhou,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200693