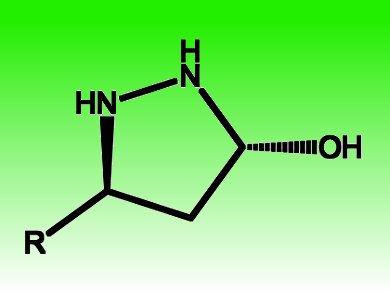
Pyrazolidines and pyrazolines represent important structural motifs in pharmaceutically active compounds. Armando Córdova, Stockholm University, Sweden, and coworkers efficiently make pyrazolidine derivatives accessible by developing a highly chemo- and enantioselective catalytic cascade transformation between di-1,2-N-protected hydrazine derivatives and α,β-unsaturated aldehydes.
The transformation is catalyzed metal-free by chiral amines and proceeds via a direct aza-Michael/hemiaminal cascade sequence. It delivers functional 3-hydroxypyrazolidine derivatives with 98–99 % ee in one step and is a direct entry to other pyrazolidine derivatives in two steps, such as 5-allylsubstituted pyrazolidines via a subsequent Lewis acid-mediated allylation reaction. Essential for 1,4-selectivity is the use of a di-1,2-N-protected hydrazine derivative, since monoprotected hydrazine as the dinitrogen source predominantly leads to hydrazone formation.
- Direct Catalytic Asymmetric Synthesis of Pyrazolidine Derivatives,
Luca Deiana, Gui-Ling Zhao, Hans Leijonmarck, Junliang Sun, Christian W. Lehmann, Armando Córdova,
ChemistryOpen 2012, 1(3), 134–139.
DOI: 10.1002/open.201200015
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.