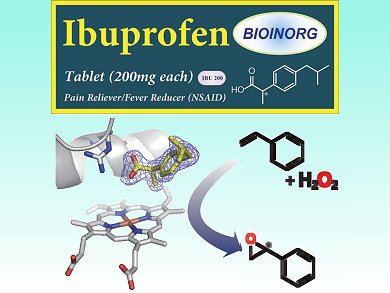
The ubiquitous heme-containing cytochrome P450 enzymes can be used as selective biocatalysts for oxidation. However, they usually require stoichiometric amounts of the expensive cofactor nicotinamide adenine dinucleotide phosphate (NAD(P)H) for the reductive activation of molecular oxygen. Hydrogen peroxide is an attractive alternative oxidant, but P450s that use H2O2 exhibit high substrate selectivity, limiting their practical use.
Yoshihito Watanabe and colleagues, Nagoya University and RIKEN Institute, Japan, have found a way to expand the substrate scope of P450-catalyzed oxidation with H2O2. They use organic acids to simulate substrate binding. The carboxylic acid group activates the H2O2 oxidant, which finally results in oxidation of the heme iron atom and generation of the active species. This “decoy” approach allows epoxidation of styrene, which cannot normally be achieved with P450 enzymes. When a single enantiomer of a chiral decoy is used, such as the common pain reliever ibuprofen, the epoxidation proceeds stereoselectively.
Further optimization of the system both in terms of decoy molecule and P450 mutant should yield higher catalytic activity and better stereoselectivity.
- Chiral-Substrate-Assisted Stereoselective Epoxidation Catalyzed by H2O2-Dependent Cytochrome P450SPα,
Takashi Fujishiro, Osami Shoji, Norifumi Kawakami, Takahiro Watanabe, Hiroshi Sugimoto, Yoshitsugu Shiro, Yoshihito Watanabe,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200250