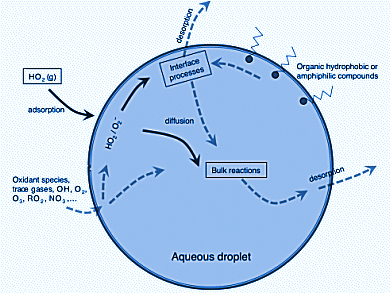
Aerosols and clouds play important roles in atmospheric chemistry, but molecular details of the process are not yet completely understood, despite many investigations carried out in the last few years. On one hand, the uptake of a compound into an aerosol or a water droplet modifies its gas-phase concentration and chemical kinetics. On the other hand, the condensed phase allows for otherwise unfeasible processes to occur in the atmosphere, ionic reactions in aqueous environments being a prototypical example.
Manuel F. Ruiz-Lopez, University of Lorraine, Vandoeuvre-les-Nancy, France, and colleagues investigated the chemical properties at the air–water interface of the atmospherically important free radicals HO2. and O2.− by means of computer simulations. Acidity, HOMO–LUMO gap, and redox potentials differ from both the bulk and also the gas phase.
This has significant ramifications for HO2 chemistry or aerosol and cloud chemistry. At the interface, two main effects influencing the reactivity can be expected:
- A decrease of ionic dissociation constant by about 1–2 units and increase of HO2 concentration with respect to the bulk.
- A change of the O2/O2– redox potential by about –0.3 V.
- Reactivity of Atmospherically Relevant Small Radicals at the Air–Water Interface,
Marilia T. C. Martins-Costa, Josep M. Anglada, Joseph S. Francisco, Manuel F. Ruiz-Lopez,
Angew. Chem., Int. Ed. 2012, 51, 5413–5417.
DOI: 10.1002/anie.201200656