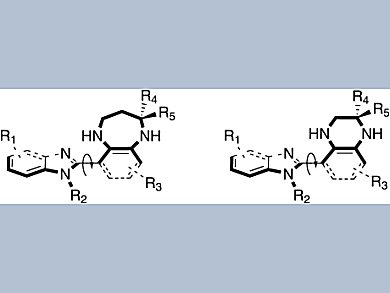
Privileged structures in drugs comprise rigid 3D arrangements of functional groups, allowing them to interact effectively with target biomolecules, such as enzymes and receptors. A group of researchers led by Chung-Ming Sun of National Chiao Tung University, Taiwan, has synthesized molecular scaffolds that each present two such privileged structures.
Starting from commercially available 4-fluoro-3-nitrobenzoic acid and 2-chloro-3-nitrobenzoic acid, the researchers prepared a 1-benzimidazolyl-2-indolyl-3-nitrobenzene derivative. This compound served as the common starting material for a variety of indolo-fused benzodiazepinyl and quinoxalinyl benzimidazoles, which were constructed by Pictet–Spengler-type heterocyclization with a variety of ketones and aldehydes.
Single-crystal analysis indicated that these scaffolds can present various substituents with distinct three-dimensional orientations, which is a promising feature for the development of new pharmaceuticals. Future screening against diverse biological targets as well as structure–activity studies should reveal the potential of this family of compounds.
- Design and Synthesis of New Biprivileged Molecular Scaffolds: Indolo-Fused Benzodiazepinyl/quinoxalinyl benzimidazoles,
Indrajeet J. Barve, Chan-Yu Chen, Deepak B. Salunke, Wen-Sheng Chung, Chung-Ming Sun,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200121