No matter if it is a giant complex, a high-rise, or an underground project, modern architecture cannot get along without concrete. The component in concrete that holds the other components together is cement. In order to control the properties of concrete, it is important to know what occurs as it hardens. German scientists have now successfully watched the first few seconds in the “life” of cement by means of X-ray diffraction. In the journal Angewandte Chemie, they explain the role of the superplasticizers added to concrete.
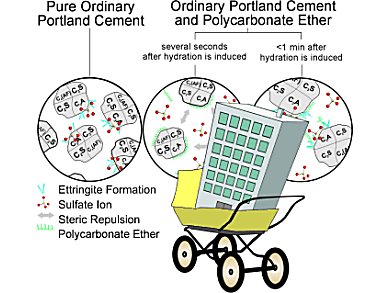
Concrete is made from sand, gravel, additives, water, and cement. Portland cement is a complex mixture of finely ground limestone, clay, sand, and iron ore—mainly calcium silicate with fractions of aluminum and iron compounds and sulfates. Once mixed with water, chemical reactions occur between the components of cement, and it solidifies and hardens. When the process is finished, it remains solid and stable, even under water. The enormous stability of concrete comes from crystalline needles that form during this process and are firmly interlocked with each other. Various additives are used to optimize the properties of concrete, including a class of superplasticizers based on polycarboxylate (PCE). These improve the flow of the concrete, making it easier to pour. The water content can be reduced to improve the concrete’s compressive strength.
“Detailed insight into the different stages of the hydration process is essential for a more complete understanding of how these processes can be effectively influenced,” explains Franziska Emmerling of the BAM Federal Institute of Materials Research and Testing in Berlin (Germany). “In particular, the phase development at the beginning of hydration is not yet well understood.” The very rapidly initiated reaction of the cement clinker component C3A (Ca3Al2O6) with sulfate (SO42-) to form ettringite (Ca6Al2(SO4)3(OH)12·26H2O) seems to be critical. By means of high-resolution X-ray diffraction experiments, Emmerling’s team has now been able to follow this reaction on the millisecond timescale. The deflections experienced by X-rays as they pass through a material provide information about its crystal structure. In order to prevent interference from any supporting material, the sample is held in suspension by acoustic waves.
This has also made it possible to clarify the function of PCE superplasticizers. Says Emmerling: “Immediately after water contacts the cement, the PCE adsorbs onto the surface of the clinker C3A; the particles remain in suspension because they then repel each other. The PCE is then gradually replaced by sulfate ions, which retards the incipient ettringite crystallization. This leaves more free water in the system, dissolving more crystalline components—the resulting concrete can thus flow for a longer period and becomes more dense.”
- First Seconds in a Building’s Life—In Situ Synchrotron X-Ray Diffraction Study of Cement Hydration on the Millisecond Timescale,
Moritz-Caspar Schlegel, Adnan Sarfraz, Urs Müller, Ulrich Panne, Franziska Emmerling,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201200993