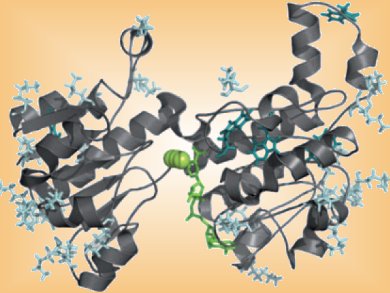
Ionic liquids (ILs) have been proposed as solvent replacements for biocatalytic applications since the beginning of the 2000s. Enzymes such as dehydrogenase do not usually work in ILs due to enzyme denaturation and low solubility of the cofactor regeneration system.
Bastien Doumèche and colleagues, Université Claude Bernard, France, have found that modification of a formate dehydrogenase by cations based on ILs allows the enzyme to maintain its activity in the presence of the IL, [MMIm][Me2PO4] (MMIm: 1-methyl-3-methyl imidazolium dimethylphosphate). The hydroxylated cations, hydroxyalkyl imidazolium, hydroxylalkyl pyrrolydinium, and cholinium, were grafted onto the enzyme through lysine coupling.
Introduction of these cations was shown to improve the stability of the enzyme by preventing the protein unfolding in the IL.
- Ionic Liquid-Inspired Cations Covalently Bound to Formate Dehydrogenase Improve its Stability and Activity in Ionic Liquids
M. Bekhouche, L. J. Blum, B. Doumèche,
ChemCatChem 2011.
DOI: 10.1002/cctc.201000390