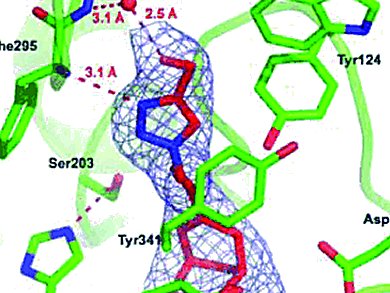
Acetylcholinesterase (AChE) plays a central role in nerve signal transmission, and blocking this enzyme’s activity was the first approach toward palliative treatment of Alzheimer’s disease (AD). Medicinal chemists have therefore focused a lot of attention over the past decades on the discovery of potent, selective, and reversible inhibitors of human AChE.
Pierre-Yves Renard at the University of Rouen, France, and his collaborators at the Institut de Biologie Structurale and Institut de Recherche Biomédicale des Armées in Grenoble, France, conducted a complete study – from rational design to validation by X-ray structure – that allowed the prediction of inhibitory potency and led to the synthesis of two huprine derivatives with excellent inhibitory activity. These molecules are among the best human AChE inhibitors reported to date.
The multifactorial and complex etiology of AD requires medicinal chemists to develop multifunctional drugs that can target two or more of the pathogenic mechanisms involved in AD. In this context, the two molecules developed by Renard and his collaborators are a good starting point for preparing new multi-target treatments for AD.
- Huprine Derivatives as Sub-Nanomolar Human Acetylcholinesterase Inhibitors: From Rational Design to Validation by X-ray Crystallography,
C. Ronco, E. Carletti, J.-P. Colletier, M. Weik, F. Nachon, L. Jean, P.-Y. Renard,
ChemMedChem 2012, 7(3).
DOI: 10.1002/cmdc.201100438