Neuraminidases (NAs) regulate interactions with the extracellular world including infection, immunity, and cell–cell adhesion. Many pathogens utilize their own NAs, making NA inhibitors an attractive target for drug discovery.
A potent inhibitor for Vibrio cholerae neuraminidase (VCNA) – one of the oldest and best studied NA involved in cholera
pathogenesis – has been developed by Hiroshi Hinou and colleagues, Hokkaido University, Japan. Mechanism-based labeling study revealed the presence of a potential target loop for VCNA inhibition. The results were then used to prepare a focused library of 187 compounds. A [2+3] cyclization (click reaction) was used to prepare derivatives of DANA (2,3-dehydro-N-acetylneuraminic acid).
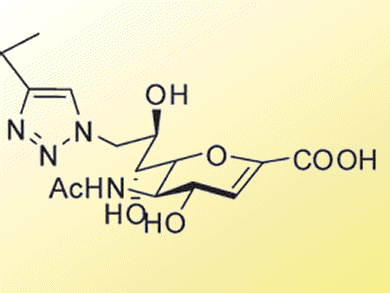
A N-trifluoroacetyl derivative of DANA, FANA, was identified as a potential inhibitor along with DANA analogue modified at the C9 position (see below). Validation studies suggested that the 9-substitution group interacted hydrophobically with the target loop moiety, adding a noncompetitive inhibition property to the DANA skeleton.
VCNA has an active site very similar to that observed in influenza (Flu) NA and Salmonella NA, meaning this two-step strategy presents a new route for the design of structurally novel anti-influenza drugs.

Image: (c) Wiley-VCH
- A Strategy for Neuraminidase Inhibitors Using Mechanism-Based Labeling Information
H. Hinou, R. Miyoshi, Y. Takasu, H. Kai, M. Kurogochi et al.,
Chem Asian J. 2011.
DOI: 10.1002/asia.201000594