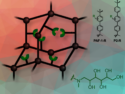
The oxidizing agent chlorine dioxide (ClO2) can be produced from chlorite ions using a manganese catalyst, according to researchers at Princeton University, USA. After adding a porphyrin manganese (III) compound to a slightly acidic solution of sodium chlorite NaClO2, “the appearance of ClO2 occurred within seconds,” Thomas P. Umile and John T. Groves write. The gaseous compound is an alternative to chlorine in paper bleaching, pathogen decontamination and water treatment.
Umile and Groves produced chlorine dioxide in a 60 % yield from a sodium chlorite solution under ambient pressure and temperature. The reaction proceeded very fast in the first two minutes after catalyst addition; then the ClO2 concentration reached a maximum. The researchers removed the product from the reaction vessel by bubbling helium through the solution and collecting the gases in water. After the reaction was finished, it could be started again by adding new sodium chlorite, “further indicating the stability of the catalysts,” as Umile and Groves write. An immobilized form of the catalyst was also active, they say.
ClO2 is endothermic, therefore quite unstable and dangerous to handle. Nowadays it is produced at large scale by reducing sodium chlorate NaClO3 in a strong acidic solution. An electrochemical oxidation of chlorite ClO2– is possible but, according to the authors, requires high energy input.
- Catalytic Generation of Chlorine Dioxide from Chlorite Using a Water-Soluble Manganese Porphyrin
T. P. Umile, J. T. Groves,
Angew. Chem. Int. Ed. 2010.
DOI: 10.1002/anie.201004482