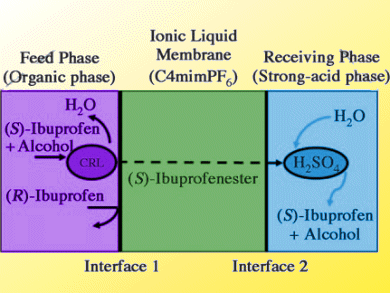
The pharmacological activity of ibuprofen, a common painkiller and anti-inflammatory drug, arises predominantly from its (S)-form. Separation of the (R)- and (S)-forms is difficult as they differ only in optical polarity.
The continuous and simultaneous reaction/separation of ibuprofen racemate has been reported by Dong-Pyo Kim and co-workers, Chungnam National University, South Korea. They use an inorganic polymer-chip device with triple-laminar flow. A feed phase with Candida rugosa lipase was used to facilitate esterification of (S)-ibuprofen due to its selective chiral recoginition and resolution. The (S)-ibuprofen ester was then transported through an ionic liquid which acted as a pseudo-membrane. Finally, conversion into (S)-ibuprofen was achieved by H2SO4 acid hydrolysis in a receiving stream.
The separated ibuprofen revealed very high chiral purity, with 99.9 % enantiomeric excess throughout the process conditions.
- Chiral Separation by a Pseudo Membrane in a Triple-Laminar Flow with a Microfluidic Contactor
T.-H. Yoon, L.-Y. Hong, D.-P. Kim,
Chem. Asian J. 2011.
DOI: 10.1002/asia.201000798