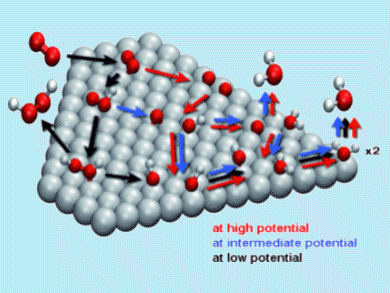
John Keith and Timo Jacob, Ulm University, Germany, have reported new insights into the mechanistic details of the oxygen reduction reaction (ORR). Their approach was based on series of DFT calculations and led the authors to develop a simplified kinetic model of the dependence of catalytic ORR on Pt(111) electrodes.
After quantifying the relevant reaction energies of three different pathways their relative contributions were compared as a function of electrode potential based on the kinetic model (see figure for the possible reaction mechanisms of the ORR on Pt(111)). Thus, they discovered that the ORR mechanism is sensitive to the applied electrode potential and other reaction conditions.
- Theoretical Studies of Potential-Dependent and Competing Mechanisms of the Electrocatalytic Oxygen Reduction Reaction on Pt(111)
J. A. Keith, T. Jacob,
Angew. Chem. Int. Ed. 2010, 49.
DOI: 10.1002/anie.201004794 - J. A. Keith, T. Jacob,
Angew. Chem. 2010, 122.
DOI: 10.1002/ange.201004794