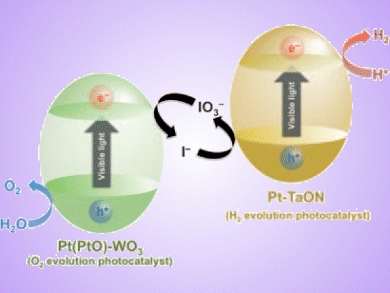
Splitting water into H2 and O2 by semiconductor photocatalysts has received much attention due to the potential for the large scale production of H2 from water using abundant solar light. But, not many semiconductors have the capability to absorb visible light and a sufficient potential for water splitting.
Ryu Abe, Hokkaido University, Japan, and co-workers present an effective photocatalytic water splitting system that mimics the two-step photoexcitation of photosynthesis in green plants. The water splitting reaction is divided into two parts, one for H2 evolution, using Pt-loaded tantalum oxynitride and another for O2 evolution, with Pt-loaded WO3. These are combined with a shuttle redox couple (IO3–/I–) in the solution.
The efficiency of overall water splitting was significantly affected by the concentration of iodide anions in the solution, as well as by the pH of the solution.
Image: (c) Wiley-VCH
- Overall Water Splitting under Visible Light through a Two-Step Photoexcitation between TaON and WO3 in the Presence of an Iodate–Iodide Shuttle Redox Mediator
R. Abe, M. Higashi, K. Domen,
ChemSusChem 2011.
DOI: 10.1002/cssc.201000333