As one of the “holy grails” in chemistry, the activation of methane has been studied for many decades. It may enable the conversion of cheap and abundant natural gas into much more valuable organic compounds. Due to the extremely high stability of methane, temperatures >700 °C are necessary to achieve high CH4 conversion efficiency and selectivity. Undesirable byproducts are always produced in traditional catalytic processes.
Gas-phase studies on the low-temperature activation of methane (or other alkanes) by clusters have attracted much interest, partly due to the possibility of revealing the mechanisms for reactions occurring on catalyst surfaces.
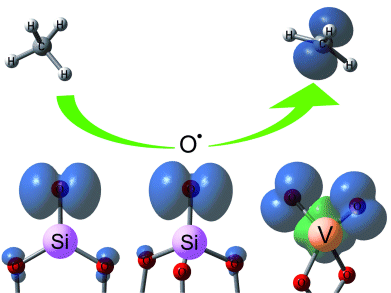
Time-of-flight mass spectrometry and density functional theory were used to study the reactions of vanadium–silicon heteronuclear oxide cluster cations with methane. S.-G. He et al., Beijing National Laboratory for Molecular Science, Beijing, China, demonstrate that the stoichiometric oxide clusters [V2O5(SiO2)1–4]+ and [(V2O5)2SiO2]+, which contain terminal-oxygen-centered radicals (Ot•) responsible for high C–H activation, are able to activate methane under near-room-temperature conditions.
- Hydrogen-Atom Abstraction from Methane by Stoichiometric Vanadium–Silicon Heteronuclear Oxide Cluster Cations
X.-L. Ding, Y.-X. Zhao, X.-N. Wu, Z.-C. Wang, J.-B. Ma, S.-G. He,
Chem. Eur. J. 2010, 16 (37).
DOI: 10.1002/chem.201001297