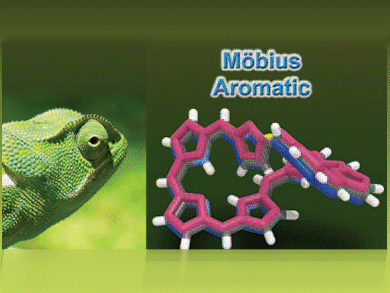
Expanded porphyrins have potential applications as anion sensors, photosensitizers, and as near-infrared (NIR) dyes. Their high structural flexibility limits their use by giving twisted molecular structures and hampering the delocalization of π-electrons.
Conformational switching between Möbius aromatic and Hückel antiaromatic congeners in expanded porphyrins has been investigated in a collaboration between Dongho Kim and co-workers at Yonsei University, South Korea, and Atsuhiro Osuka at Kyoto University, Japan. This switching phenomenon has been optically identified by using solvent- and excitation-wavelength-dependent time-resolved absorption measurements. From the photophysical properties of these porphyrins in conjuction with theoretical calculations they predict that the molecular aromaticity is sensitive to surrounding environments in expanded porphyrinoid systems.
This knowledge could help develop new ways of controlling the molecular conformations of expanded porphyrins.

- Solvent Dependent Aromatic versus Antiaromatic Conformational Switching in meso-(Heptakis)pentafluorophenyl [32]Heptaphyrin
M.-C. Yoon, J.-Y. Shin, J. M. Lim, S. Saito, T. Yoneda, A. Osuka, D. Kim,
Chem. Eur. J. 2011, 17, 6707.
DOI: 10.1002/chem.201003736