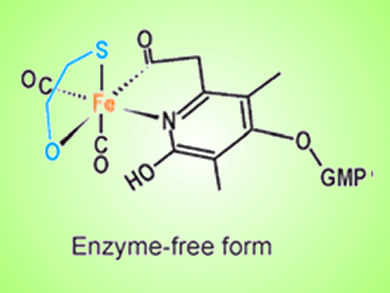
A research team led by Seigo Shima, Max-Planck-Institute for Terrestrial Microbiology, Germany, has successfully applied iron-chromophore circular dichroism (CD) spectroscopy to probe the mechanism of an enzyme that binds hydrogen gas, thereby confirming predictions from crystal structures of the protein.
[Fe]-hydrogenase, which contains a unique iron cofactor (FeGP cofactor), catalyzes the reversible hydrogenation of methenyltetrahydrometanopterin (methenyl-H4MPT+) with H2 to methylene-H4MPT (see scheme). The CD data support the hypothesis that the binding of methenyl/methylene-H4MPT induces a conformational change that closes the active-site cleft of [Fe]-hydrogenase to form the intact active site.

- Iron-Chromophore Circular Dichroism of [Fe]-Hydrogenase: The Conformational Change Required for H2 Activation
S. Shima, S. Vogt, A. Göbels, E. Bill,
Angew. Chem. Int. Ed. 2010, 49.
DOI: 10.1002/anie.201006255 - S. Shima, S. Vogt, A. Göbels, E. Bill,
Angew. Chem. 2010, 122.
DOI: 10.1002/ange.201006255