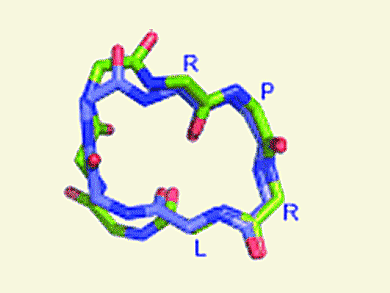
Apelin is a recently discovered peptide that binds to the apelin (or APJ) G-protein-coupled receptor. Apelin-13 (NH2-QRPRLSHKGPMPF-COOH), one of several cleavage products of the proprotein form of the apelin gene product, is a vasoactive peptide and is one of the most potent endogenous inotropic agents known so far. After having conducted extensive replica-exchange molecular dynamics and competition binding experiments, N. J. Maximilian Macaluso and Robert C. Glen at the University of Cambridge report the design and evaluation of head-to-tail cyclized analogues of the apelin-13 peptide in the journal ChemMedChem.
“The receptor-bound conformation of apelin, if known, would greatly facilitate the rational design of novel agonists and antagonists at APJ,” says Glen. “Interest in apelin as a drug target has greatly increased with recognition of its role in cardiovascular disease, metabolic syndrome, and as a co-receptor for HIV infection.”
This combined in silico and in vivo approach revealed that peptides promoting a β turn at the RPRL motif toward the N terminus of apelin-13 show affinity for the APJ receptor, whereas those without this RPRL turn exhibit almost no binding at APJ. This is a critical step in understanding the APJ receptor binding site, which has not yet been identified. The study lays the foundation for not only further development of truncated cyclic peptide analogues of apelin-13, but also the development of non-peptide mimetics.
- Exploring the RPRL Motif of Apelin-13 through Molecular Simulation and Biological Evaluation of Cyclic Peptide Analogues
N. J. M. Macaluso, R. C. Glen,
ChemMedChem 2010, 5.
DOI: 10.1002/cmdc.201000061