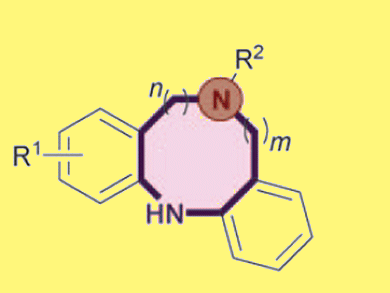
David Spring and co-workers, University of Cambridge, UK, have described the synthesis of rare nitrogen-linked seven-, eight- and nine-membered biaryl ring systems. The team uses an activated intramolecular copper species to facilitate (aryl)–N bond formation, closing the acyclic precursor.
An excellent yield (91 %) of eight-membered N-linked biaryl was obtained by use of a combination of copper(I) iodide, Cs2CO3, and ethylene glycol which acted both as solvent and ligand. No evidence of competing intermolecular couplings was seen, despite the relatively high concentrations employed. A range of electron donating and withdrawing substituents on the aniline-based ring portion were tolerated, leading to a broad substrate scope.
The process is technically simple, and uses cheap starting materials with mild conditions to give biologically valuable products that are difficult to synthesize by other methods.

Images: (c) Wiley-VCH
- Novel and Efficient Copper-Catalysed Synthesis of Nitrogen-Linked Medium-Ring Biaryls
J. L. Kenwright, W. R. J. D. Galloway, D. T. Blackwell, A. Isidro-Llobet, J. Hodgkinson et al.,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201002093