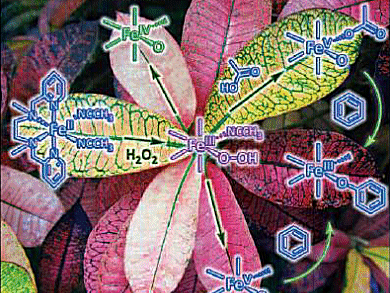
Until now, no intermediates have been observed in C–H oxidations with hydrogen peroxide catalyzed by iron complexes.
Elena Rybak-Akimova and Olga Makhlynets, Medford MA, USA, have performed detailed kinetic and mechanistic studies on oxidations with hydrogen peroxide catalyzed by [Fe(bpmen)(CH3CN)2][ClO4]2 (bpmen=N,N’-dimethyl-N,N’-bis(2-pyridylmethyl)ethane-1,2-diamine).
It was found that that FeIII(OOH) produces the reactive oxidant in the rate-limiting, acid-assisted heterolytic cleavage of the O
- Aromatic Hydroxylation at a Non-Heme Iron Center: Observed Intermediates and Insights into the Nature of the Active Species,
Olga V. Makhlynets, Elena V. Rybak-Akimova,
Chem. Eur. J. 2010, 16 (47).
DOI: 10.1002/chem.201002577