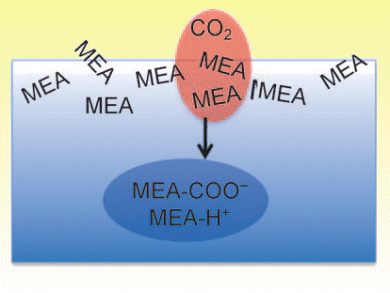
Aqueous amine-based solutions are the cheapest currently employed solvents to capture CO2 emitted from industrial processes. CO2 capture involves flowing flue gas through a solution containing a relatively nontoxic compound such as monoethanolamine (MEA) that reacts selectively with CO2. Understanding the properties that are essential for efficient CO2 capture will be of great relevance for the development of more energy-efficient processes and the design of capture solutions.
John Hemminger and colleagues, University of California — Irvine, USA, have studied the role of the solution–gas interface in this reaction as it is of key importance for properly modeling absorption kinetics. Their results support a model in which CO2 encounters pure MEA at the solution surface, and the reaction products and protonated MEA — formed by MEA reacting with CO2 and with water — move into the bulk of the solution once they are formed.
This implies that a higher concentration of neutral MEA at the solution surface would increase the scavenging rate for CO2, and allow more efficient CO2 capture.
- CO2 Capture in Amine-Based Aqueous Solution: Role of the Gas–Solution Interface
T. Lewis, M. Faubel, B. Winter, J. C. Hemminger,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201101250