For fuel-cell applications, ethanol is becoming a more attractive fuel than methanol or hydrogen as it is less toxic than methanol, easier to handle than hydrogen, and can be produced in great quantities from biomass. However, incomplete oxidation leads to a more complex range of reaction intermediates and products than for H2 or methanol.
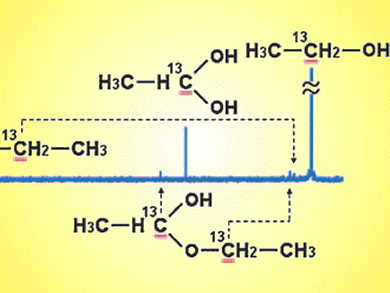
Oc Hee Han and colleagues, Korea Basic Science Institute, have used 13C liquid-state NMR spectroscopy to identify and quantify the reaction products present in the liquid anode exhaust of direct ethanol fuel cells. The amounts of acetic acid (AA), acetaldehyde (AAL), and ethane-1,1-diol (ED) were shown to vary depending on the catalyst and operating potential.
Addition of Ru or Sn to Pt/C anode increases current density, mainly because of enhanced acetic acid production. The potential dependences of products suggest that the water dissociation reaction was not the only major controlling factor for the AA and ED production, and AAL and ED behave as intermediates to be converted into AA.
- Catalytic Reactions in Direct Ethanol Fuel Cells
I. Kim, O. H. Han, S. A. Chae, Y. Paik, S.-H. Kwon, K.-S. Lee, Y.-E. Sung, H. Kim,
Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201005745