Synthesis of Complex Organic Molecules
The synthesis of complex organic molecules, especially natural products, is an art that demands:
- considerable creativity in a conceptual deconstruction of the target molecule,
- much preparative organic knowledge in the development of a synthetic strategy that promises success, and finally
- the practical skill necessary to succeed in the actual transformative process.
The esthetics and elegance of such a synthetic sequence is revealed only rarely to the nonorganic chemist, as – generally speaking – the complex structural formulas involved have a tendency to utterly baffle the untrained observer. This is in fact hardly surprising, since every work of art, whether it be an abstract painting, a piece of music, a poem, or in this case an organic synthesis, unveils itself to the layman only after a well-grounded and at the same time intelligible introduction to the work in question.
Here, we attempt to demonstrate this with respect to the 1934 Reichstein–Grüssner vitamin C synthesis. The effort seems particularly worthwhile, as the “protective group” technique, an interesting biotechnological reaction step, and finally an uncanny stereochemical trick have all been so masterfully incorporated.
Even today, more than 70 years after its publication, this synthesis (with a few minor changes) continues to be employed on an industrial scale, producing annually more than 60,000 t of vitamin C. After numerous optimizations of the individual reaction steps, the yield of each now exceeds 90 %, and glucose is transformed into vitamin C in an overall yield of greater than 50 %.

Figure 1. The Reichstein–Grüssner vitamin C synthesis.
Synthesis of Vitamin C
In the interest of chemical/esthetic appreciation let us consider the overall synthesis one step at a time.
Synthetic Step a
Starting with glucose (3), the aldehyde group CHO is first reduced to a primary alcohol (CH2OH). This is accomplished in high yield by a catalytic reduction with hydrogen under high pressure. The result is the sugar-substitute sorbitol (4), often utilized by diabetics.
Synthetic Step b
With the aid of microorganisms, e.g., Acetobacter xylinum or Gluconobacter oxydans, sorbitol (4) is next oxidized with oxygen. In this case the OH group at C5 is oxidized selectively into a keto group. What results is ketohexose L-sorbose (5).
According to the rules governing the use of a Fischer projection for naming compounds [1], the molecule must first be arranged so that the most highly oxidized end of its chain appears at the top. Horizontal bonds are now understood to come toward the viewer, out of the paper. The stereogenic carbon center farthest down the chain is the one used to establish assignment of an L or D classification to the molecule, depending on whether its OH appears on the right or the left. In other words: for a proper Fischer projection, structure 5 must first be rotated 180°. This leads us ultimately to the more complete identification for 5 (L-sorbose). For clarification, we have indicated which C atom was originally the C1 atom in glucose with an asterisk.
Synthetic Step c
Unfortunately, it is not possible to selectively oxidize the terminal, primary alcohol group in L-sorbose (5) into a carboxylic acid, because, regardless of which oxidizing agent is chosen, the other hydroxy groups are oxidized as well. For that reason, a synthetic detour is now required, in which four of the five hydroxy groups are first protected against oxidation.
Equilibrium d
As is true of most hexoses, L-sorbose exists in aqueous solution as an equilibrium mixture between the open-chain form (5) and a cyclic hemiacetal (7).
Synthetic Step e
As suggested above, what comes next is the protection of four hydroxy groups, all in a single step. At this point, Reichstein discovered an ideal, economic, and environmentally friendly protective group for this purpose: acetone. The keto group of acetone can react with two adjacent hydroxy groups to give a cyclic acetal, and with a skillful choice of the reaction conditions, two molecules of acetone can be induced to protect against oxidation of all four of the hydroxy groups in question. What results is diacetonide 8.
Synthetic Step f
Oxidation of 8 with hypochlorite or air over palladium or platinum on charcoal produces a good yield of the carboxylic acid 9.
Synthetic Step g
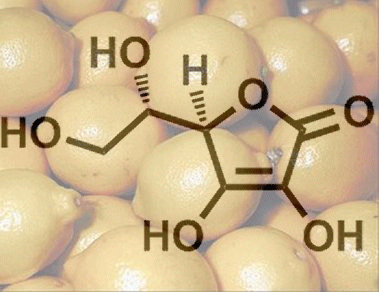
In the presence of acid, acetone is cleaved off from the double acetal. In other words, both protective groups can be removed at once to yield 2-keto-L-gulonic acid (6). This name – often encountered – unfortunately does not conform to standard rules of nomenclature. The correct CAS name would be L-threo-2,3-hexodiulosonic acid, but the systematic IUPAC name is ((3S,4R,5S)-3,4,5,6-tetrahydroxy-2-oxohexanoic acid.
Synthetic Step h
Finally, there is an acid-catalyzed tautomerization of 6 by way of 10 to the desired L-ascorbic acid (1), shown here in its cyclic form.
Admittedly, for the nonorganic chemist this synthesis probably remains something of a muddle. Still, you should remember that ascorbic acid is, after all, a natural product, one that contains two stereogenic carbon atoms, and it has been obtained (in remarkably few steps!) from glucose by effectively turning the latter “on its head”.
Today, with a few minor modifications, this process is still able to do the job commercially, and in an overall yield of better than 50 %. Hats off to Reichstein and Grüssner for their impressive synthetic strategy, but also to the engineers and facility designers who made possible the preparation of batches in enormous fermenters, and who perfected the synthetic process to such a degree that today vitamin C is available cheaply to everyone.
References
[1] K. Roth, Chem. Unserer Zeit 2002, 36, 390. DOI: 10.1002/1521-3781(200212)36:6<390::AID-CIUZ390>3.0.CO;2-N
This is a part of the article:
- Vitamin C Deficiency – Part 2,
Klaus Roth, Sabine Streller,
ChemViews Mag. 2014.
DOI: 10.1002/chemv.201400005