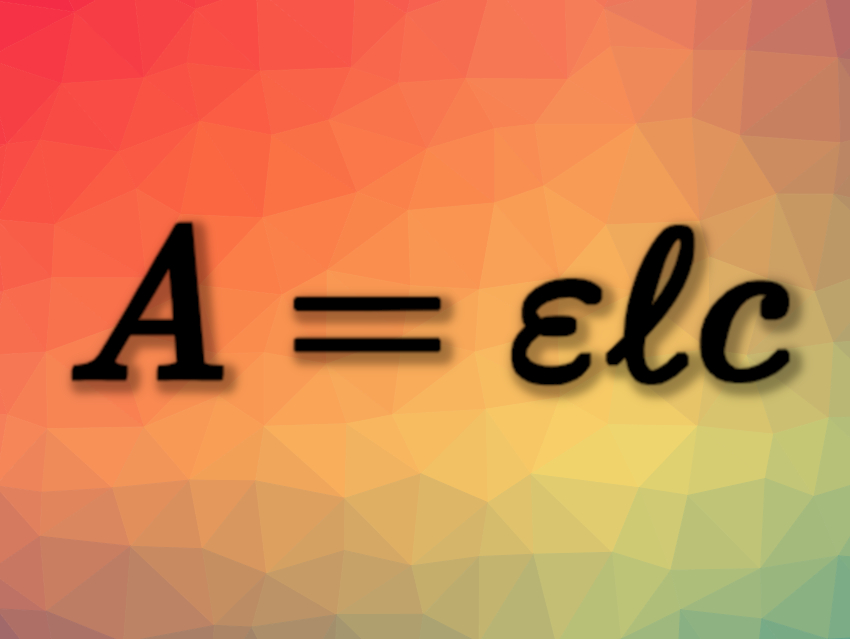August Beer was born on July 31, 1825, in Trier, Germany. He studied mathematics and natural sciences at the University of Bonn, Germany, and obtained his Ph.D. there in 1848 under the supervision of Julius Plücker.
In 1850, Beer became Lecturer at the University of Bonn. In 1855, he was appointed Associate Professor of Mathematics there, and in 1857, he was promoted to Full Professor. August Beer died on November 18, 1863, at the age of only 38.
Beer worked on many aspects of physics, with a focus on optics and light absorption. He is best known for his contributions to the Beer–Lambert–Bouguer law, which relates the absorption of light to the properties of the material through which the light passes.
 |
|
Photometer devised by Beer. |
Beer–Lambert–Bouguer Law
The Beer–Lambert–Bouguer law is named after August Beer, Johann Heinrich Lambert, and Pierre Bouguer, and is also known as the Beer–Lambert law.
Bouguer first discovered parts of the law, published in his Essai d’optique sur la gradation de la lumière (“Essay on optics on the gradation of light”) in 1729 [1]. In this work, he showed, e.g., how the intensity of light decreases after passing through a given part of the atmosphere.
Lambert published his work Photometria, sive de mensura et gradibus luminis, colorum et umbrae (“Photometry, or the measurement and degrees of light, colors, and shadows”) in 1760 [2]. Lambert showed that the loss of light intensity after passing through a material is proportional to the path length. In mathematical terms, this means:
A ∝ ℓ
(A = absorbance, ℓ = path length)
In 1852, Beer discovered another part of the puzzle [3]: He found that the absorbance is also proportional to the concentration of the solute when light passes through a solution, i.e.:
A ∝ ℓc
(A = absorbance, ℓ = path length, c = concentration)
The proportionality constant is called the molar absorption coefficient of the light-absorbing species, giving the Beer–Lambert–Bouguer law:
A = ɛℓc
(A = absorbance, ɛ = molar absorption coefficient, ℓ = path length, c = concentration)
This law is useful in quantitative analytical chemistry, where the concentration of a solution can be determined by measuring its absorbance over a fixed path length at a fixed wavelength of light when the molar absorption coefficient at this wavelength is known. The law can break down at very high concentrations, for highly scattering materials, or for very intense light.
August Beer is the answer to Guess the Chemist (125).
References
[1] Essai d’optique sur la gradation de la lumière,
P. Bouguer,
Claude Jombert, Paris, France, 1729.
[2] Photometria sive de mensura et gradibus luminis, colorum et umbrae,
J. H. Lambert,
Eberhardt Klett, Augsburg, Germany, 1760.
[3] Bestimmung der Absorption des rothen Lichts in farbigen Flüssigkeiten,
A. Beer,
Ann. Phys. 1852, 162, 78–88.
https://doi.org/10.1002/andp.18521620505
Sources
- Beer, August,
E. Lommel,
Allgemeine Deutsche Biographie (ADB) 1875, 2, 245. - Beer, August,
C. Graf von Klinckowstroem,
Neue Deutsche Biographie (NDB) 1953, 1, 734.