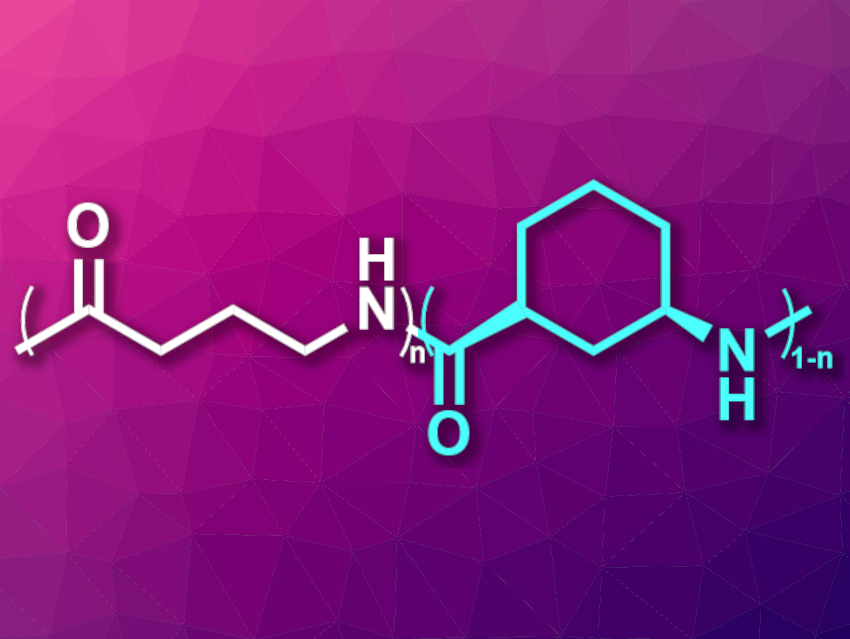
Nylons are polyamides that are often used, e.g., in textiles and fishing gear. There has been research into biobased polyamides to make these materials more sustainable, but the recycling of nylons is still an issue and not very common. The chemical recycling of polymers back to the monomers is one approach to recycling plastics. However, it can be challenging to find monomers for polyamides that combine chemical recyclability with good stability and processability. Nylon 4 (pictured in white), for example, which is based on a five-membered lactam, provides chemical recyclability but degrades when it melts, which hinders processing.
Eugene Y.-X. Chen, Colorado State University, Fort Collins, USA, and colleagues have developed a hybrid nylon 4/6 (pictured in cyan), based on the bicyclic lactam 6-azabicyclo[3.2.1]octan-7-one, and copolymerized it with nylon 4 to obtain a chemically recyclable nylon with useful properties. The hybrid nylon 4/6 showed high thermal stability up to 357 °C, but without melting below the onset of degradation. It can be chemically recycled in the presence of a ZnCl2 catalyst.
The team then prepared copolymers of nylon 4/6 in the hopes of retaining the thermal stability and chemical recyclability, but allowing melt processing. They found that a 50/50 copolymer of nylon 4/6 and nylon 4 (pictured) is a mechanically strong, optically clear, and thermally stable polyamide material. This copolymer also provides good processability and chemical recyclability. According to the researchers, the possible biodegradation of the copolymer is also being investigated.
- Redesigned Hybrid Nylons with Optical Clarity and Chemical Recyclability,
Robin M. Cywar, Nicholas A. Rorrer, Heather B. Mayes, Anjani K. Maurya, Christopher J. Tassone, Gregg T. Beckham, Eugene Y.-X. Chen,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.1c12611