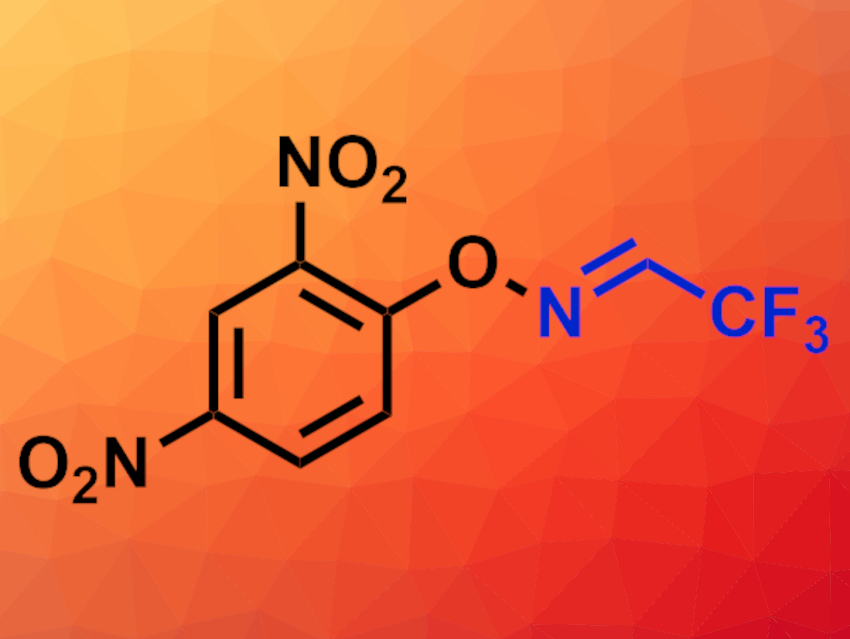
The introduction of trifluoromethyl groups into organic compounds can be useful, e.g., in pharmaceutical or materials chemistry. Trifluoroacetonitrile (CF3CN), for example, can be used for the synthesis of trifluoromethylated nitrogen-containing heterocyclic compounds. However, CF3CN is a hard-to-handle, toxic gas, which limits its use.
Yangjie Huang, Minjiang University, Fuzhou, China, Zhiqiang Weng, Minjiang University and Fuzhou University, China, and colleagues have synthesized a convenient precursor for generating trifluoroacetonitrile in situ under mild conditions. The team prepared a 2,2,2-trifluoroacetaldehyde O-(aryl)oxime (pictured) from commercially available O-(2,4-dinitrophenyl)hydroxylamine via a condensation with trifluoroacetaldehyde hydrate under acidic conditions.
The team found that the synthesized precursor released CF3CN in quantitative yield under mildly basic conditions. The generated CF3CN could be used, for example, to prepare different 5-trifluoromethyl-1,2,4-oxadiazoles via reactions with a range of in–situ-generated nitrile oxides. 3-Aryl-5-(trifluoromethyl)-2,3-dihydro-1,2,4-oxadiazoles were also prepared via reactions of CF3CN with aryl-substituted N–tert-butyl nitrones.
- 2,2,2-Trifluoroacetaldehyde O-(Aryl)oxime: A Precursor of Trifluoroacetonitrile,
Bo Lin, Yunfei Yao, Yangjie Huang, Zhiqiang Weng,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00637