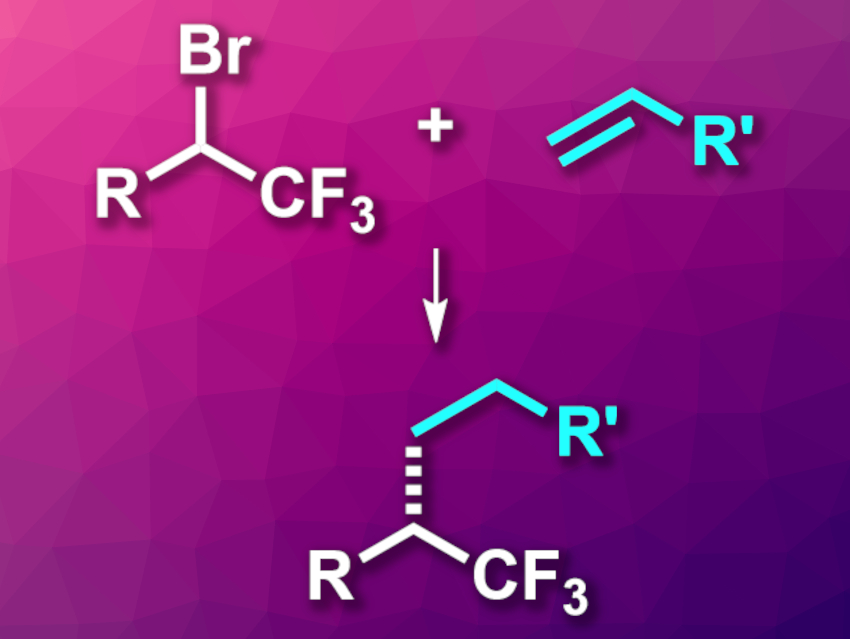
Fluorinated functional groups, such as the commonly employed trifluoromethyl (CF3) group, can be useful in pharmaceutical chemistry to improve the properties of drug candidates. Methods for the synthesis of chiral compounds containing CF3 groups can be interesting research targets in this context. Existing approaches often require additional functional groups or are limited in scope.
Guobin Ma, Weiqi Tong, Shanghai University, China, Fan Wu, Ningbo University, Ningbo, China, and colleagues have developed a nickel-catalyzed enantioconvergent hydroalkylation of olefins using trifluoromethylated α-alkyl halides for the synthesis of chiral trifluoromethylated alkanes (pictured). The team used NiBr2·diglyme (diglyme = bis(2-methoxyethyl) ether) as the catalyst together with a chiral bis(oxazoline) ligand, trimethoxysilane as a hydride source, Na2CO3 as a base, and glyme as the solvent. The reactions were performed at –10 °C.
Using this protocol, the researchers reacted a range of alkenes or functionalized alkene derivatives with a variety of CF3-functionalized alkyl bromides. The desired products were obtained in moderate to good yields and with high enantioselectivities. The team also successfully functionalized different natural products and drug derivatives. The reaction proceeds under mild conditions and shows good functional group tolerance. According to the researchers, the work could be particularly useful for the rapid construction of complex, chiral, CF3-containing molecules for drug design and screening.
- Diverse Synthesis of Chiral Trifluoromethylated Alkanes via Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Unactivated Olefins,
Wenqing Guo, Li Cheng, Guobin Ma, Weiqi Tong, Fan Wu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c00148