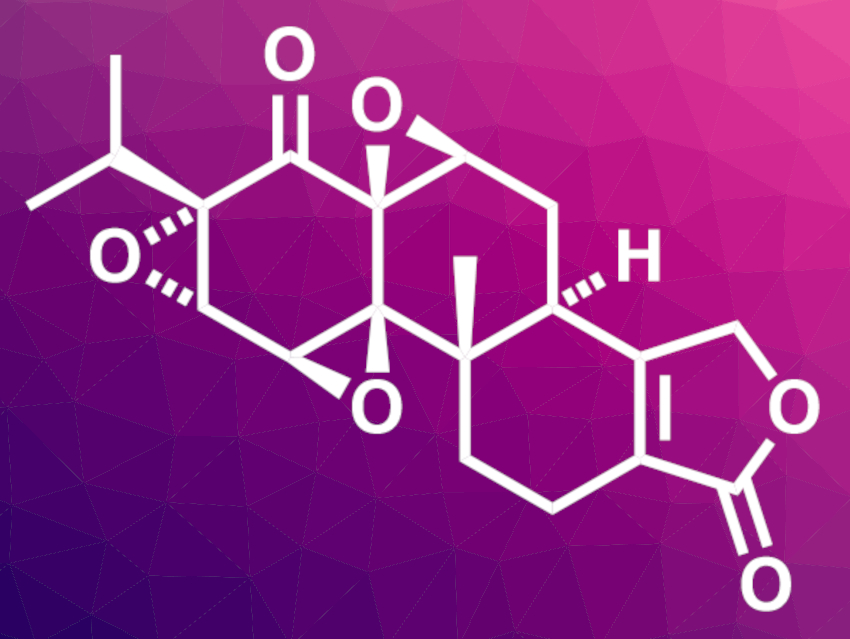
(−)-Triptolide is found in extracts of Tripterygium wilfordii Hook F, a vine plant that is used in traditional Chinese medicine. The compound has been studied for its pharmaceutical effects and could be promising for its antiflammatory and anticancer activities. A scalable synthesis of (−)-triptolide and its analogues, such as (−)-triptonide (pictured), could, thus, be useful for drug development.
Tuoping Luo, Peking University, Beijing, China, and Shenzhen Bay Laboratory, Guangdong, China, and colleagues have developed an efficient and scalable total synthesis of (−)-triptonide. The approach is based on a metal-catalyzed hydrogen atom transfer (MHAT)-initiated radical cyclization. The team started from (R)-(−)-Taniguchi lactone, which underwent a cross olefin metathesis to obtain the first fragment of the product. A second fragment was prepared from a phenol derivative via a Grignard reaction and PBr3 treatment to obtain an allylic bromide. The two fragments were coupled and converted to an aldehyde intermediate.
This intermediate was cyclized using Co(TPP) as a catalyst (TPP = tetraphenylporphyrin) under blue LED irradiation in a visible-light-promoted MHAT-initiated radical reaction. Further steps, including an aldol-type cyclization and multiple epoxidations, led to the desired (−)-triptonide. The synthesis requires eight steps (longest linear sequence). (−)-Triptonide can be easily converted to (−)-triptolide, allowing for further studies of the biological activities of both compounds.
- Scalable Total Synthesis of (−)-Triptonide: Serendipitous Discovery of a Visible-Light-Promoted Olefin Coupling Initiated by Metal-Catalyzed Hydrogen Atom Transfer (MHAT),
Xianhe Fang, Nan Zhang, Si-Cong Chen, Tuoping Luo,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.1c12525